INSUBCONTINENT EXCLUSIVE:
Depending on which study you believe, the wearable and digital health market could be worth anywhere from $30 billion to nearly $90 billion
in the next six years.If the numbers around the size of the market are a moving target, just think about how to gauge the validity and
efficacy of the products that are behind all of those billions of dollars in spending.Andy Coravos, the co-founder of Elektra Labs,
certainly has.Coravos, whose parents were a dentist and a nurse practitioner, has been thinking about healthcare for a long time
After a stint in private equity and consulting, she took a coding bootcamp and returned to the world she was raised in by taking an
internship with the digital therapeutics company Akili Interactive.Coravos always thought she wanted to be in healthcare, but there was one
thing holding her back, she says
The stint at Akili led to a position at the United States Food and Drug Administration as an entrepreneur in residence, which led to the
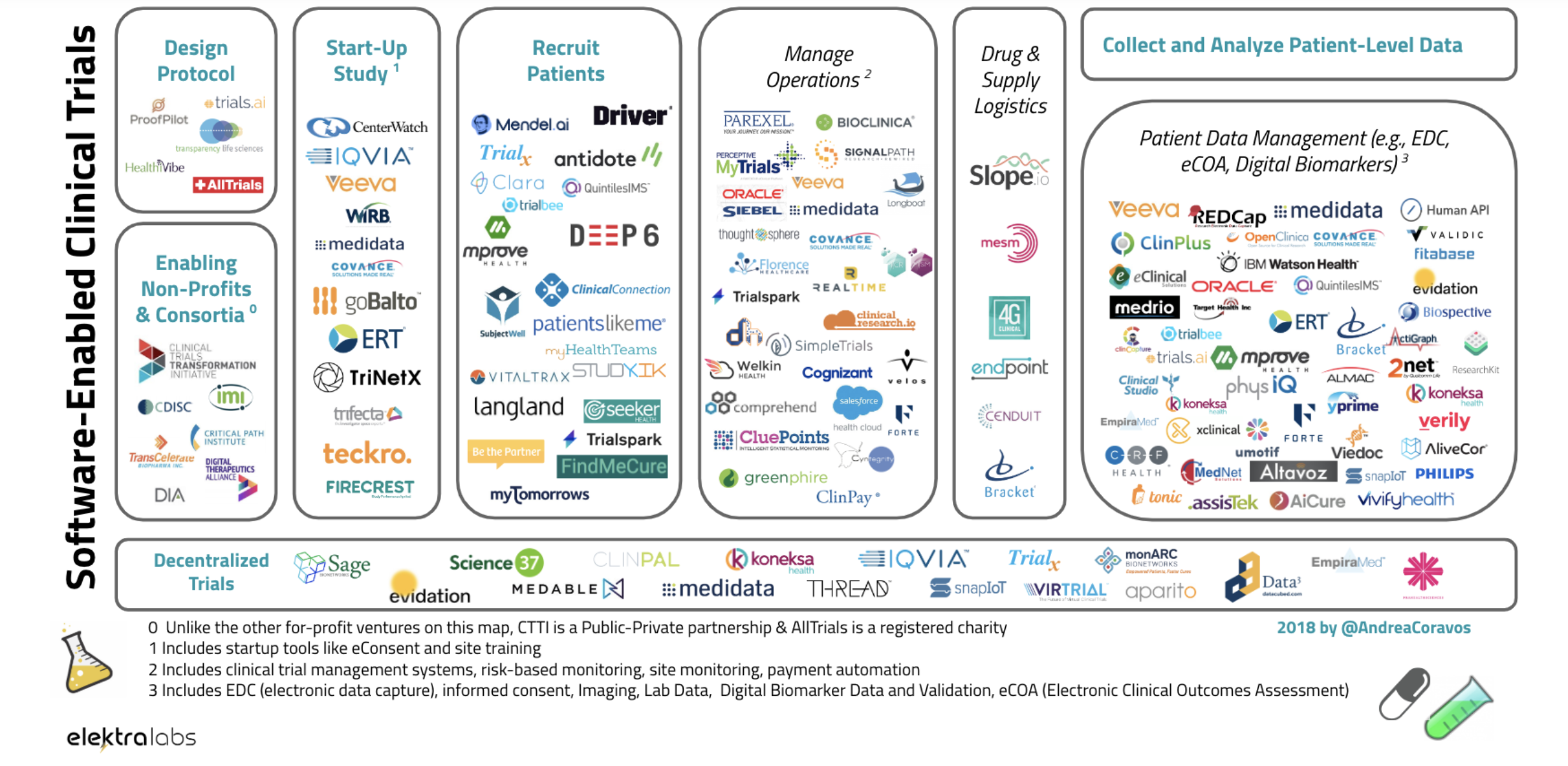
creation of Elektra Labs roughly two years ago.Now the company is launching Atlas, which aims to catalog the biometric monitoring
technologies that are flooding the consumer health market
fibrillation detection algorithm on the Apple Watch and the ActiGraph activity monitors
And big pharma companies like Roche, Pfizer and Novartis have been investing in these technologies to collect digital biomarker data and
lot of oversight that still needs to be done, and consumers and pharmaceutical companies need to have a source of easily accessible data
accelerated adoption of connected tools in both clinical trials and routine care
prepare, and dispense drug components, our healthcare system needs infrastructure to review, prepare, and dispense connected technologies
But Atlas is clearly the first pillar that the digital therapeutics industry needs as it looks to supplant pharmaceuticals as treatments for
some of the largest and most expensive chronic conditions (like diabetes).Coravos and here team interviewed more than 300 professionals as
they built the Atlas toolkit for pharmaceutical companies and other healthcare stakeholders seeking a one-stop shop for all their digital
Like a drug label, or nutrition label, Atlas publishes labels that highlight issues around the usability, validation, utility, security and
data governance of a product.In an article in Quartz earlier this year, Coravos made her pitch for Elektra Labs and the types of things it
would monitor for the nascent digital therapeutics industry
It includes the ability to handle adverse events involving digital therapies by providing a single source where problems could be reported;
a basic description for consumers of how the products work; an assessment of who should actually receive digital therapies, based on the
with undisclosed pharmaceutical companies to map out the digital therapeutic environment and identify companies that might be appropriate
modern sensing technologies can track and how effective different products are at providing those measurements
The company is also on the lookout for peer-reviewed published research or any clinical trial data about how effective various digital
products are.Backing Coravos and her vision for the digital pharmacy of the future are venture capital investors, including Maverick
Ventures, Arkitekt Ventures, Boost VC, Founder Collective, Lux Capital, SV Angel and Village Global.Alongside several angel investors,
including the founders and chief executives from companies including: PillPack, Flatiron Health, National Vision, Shippo, Revel and Verge
Indeed, Coravos believes that the two go hand-in-hand
As privacy issues proliferate across the internet, Coravos believes that the same troubles are exponentially compounded by
Terms of service that gain user consent one time, upon sign-up, are no longer sufficient
We need better social contracts that have informed consent baked into the products themselves and can be adjusted as user preferences change
over time.We need to ensure that the industry has strong ethical underpinning as it brings these monitoring and surveillance tools into the
software-driven medical products are poised to change healthcare delivery